Human iPSC-derived Oligodendrocyte Progenitor Cells (OPCs)
- Specification
- Background
- Scientific Data
- Q & A
- Customer Review
Within the central nervous system (CNS) oligodendrocyte progenitor cells (OPCs) function as critical cells that generate myelin sheath. The myelin sheath serves as a lipid layer around neuronal axons which enables quick transmission of nerve signals. OPCs perform critical functions in myelination and play significant roles in several neurodegenerative diseases. For instance, OPC dysfunction in disorders like multiple sclerosis results in disrupted nerve conduction and neuronal destruction. Additionally, OPCs respond to inflammatory mediators while they participate in both immune regulation and pathological processes during inflammatory responses.
iPSC-derived OPCs have been widely utilized in research models for multiple neurodegenerative diseases. Researchers utilize these cells in Alzheimer's disease (AD), Parkinson's disease (PD), and MS models to explore how these diseases develop and to test possible treatments. Furthermore, iPSC-derived OPCs demonstrate significant potential for advancing neural repair treatments and regenerative medical applications. Research shows that these cells can repair myelin damage while restoring neurological function. In spinal cord injury models iPSC-derived OPCs show the ability to move to lesion locations and create myelin sheaths which enhance motor abilities. Research is underway to explore these cells as potential treatments for hereditary demyelinating disorders including Canavan disease. Gene-editing technologies to reduce immunogenicity allow these cells to serve as potential "off-the-shelf" cell therapy solutions.
HUVECs Promote OPC (iPSC-derived) Proliferation, Migration, Survival, and Neuronal Axonal Growth In Vitro
Spinal cord injury (SCI) results in severe sensory and motor deficits due to demyelination and axonal degeneration. Spontaneous remyelination by endogenous oligodendrocytes is insufficient to repair this damage. Li's team explored the potential of co-transplanting iPSC-derived oligodendrocyte progenitor cells (OPCs) and human umbilical vein endothelial cells (HUVECs) to enhance functional recovery post-SCI.
During days 35–39 of differentiation, the transplanted hiPSC-GFP-OPCs into a nude rat SCI model. The number of OPC spheres, mainly containing OLIG2-positive cells, was consistent between days 20 and 30 of differentiation. However, with HUVEC conditioned medium (CM), the number of spheres increased significantly compared to cultures without CM (Fig. 1a, b). On day 65, they placed 3.5 × 104 O4+ sorted and unsorted OPCs into transwell inserts and 7 × 104 HUVECs in wells for migration assays (Fig. 1c). OPC purity was confirmed after 3 days of culture (Fig. 1d). HUVECs substantially increased cell migration after 4 hours (Fig. 1e-f and Fig. 2a). Co-culturing OPC spheres with HUVECs greatly enhanced axon growth, demonstrated by longer axons with Tuj1 staining on day 3 compared to controls (Fig. 1g, h). Similar results were seen with ESC-derived OPCs (Fig. 2b and c). On day 45, without HUVECs, axon growth and cell survival were hindered (Fig. 2d). These findings suggest HUVECs support OPC proliferation, migration, survival, and neuronal axon growth.
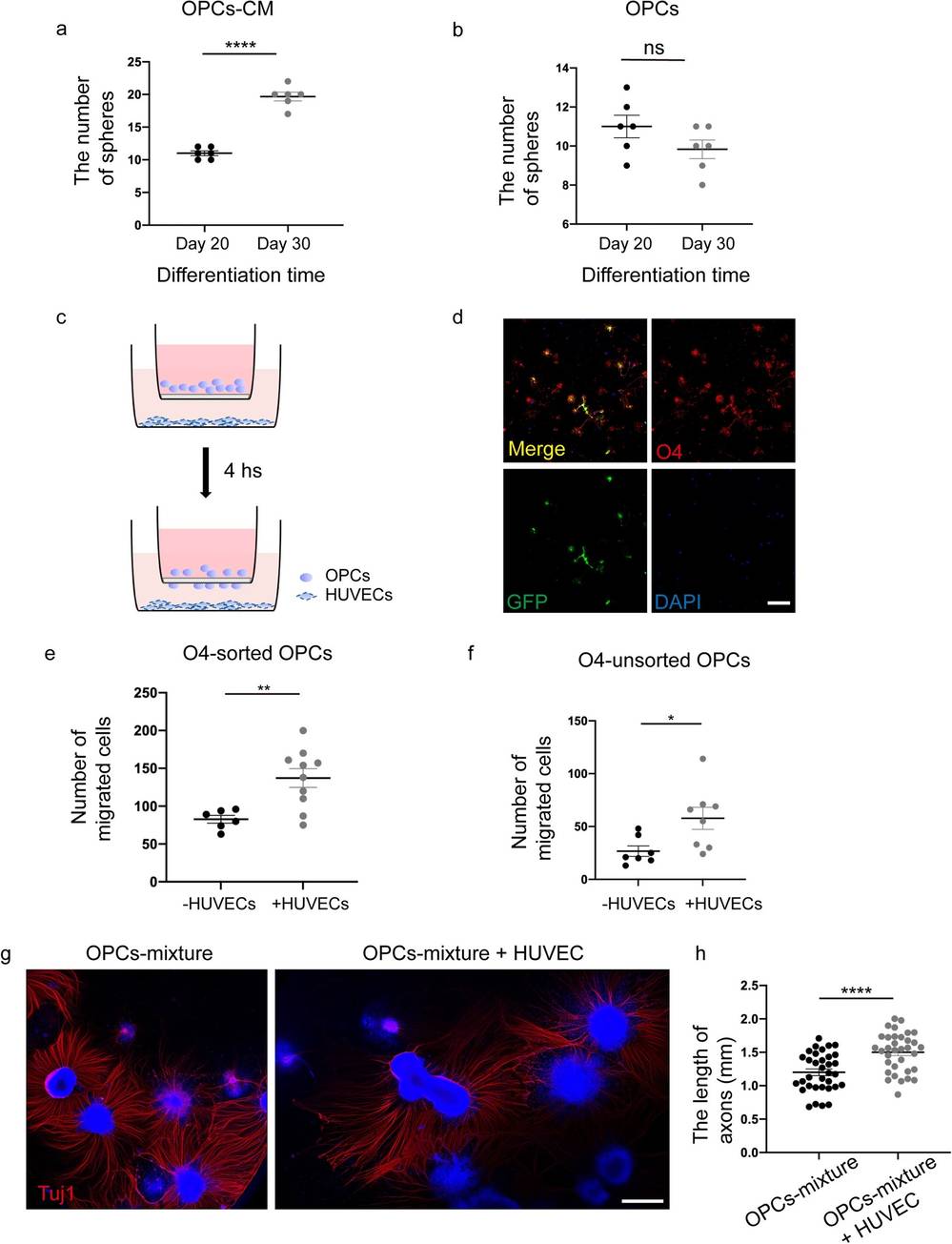 Fig. 1. HUVECs promote OPCs proliferation, migration, and neuronal axonal growth in vitro (Li Q, Liu S, et al., 2024).
Fig. 1. HUVECs promote OPCs proliferation, migration, and neuronal axonal growth in vitro (Li Q, Liu S, et al., 2024).
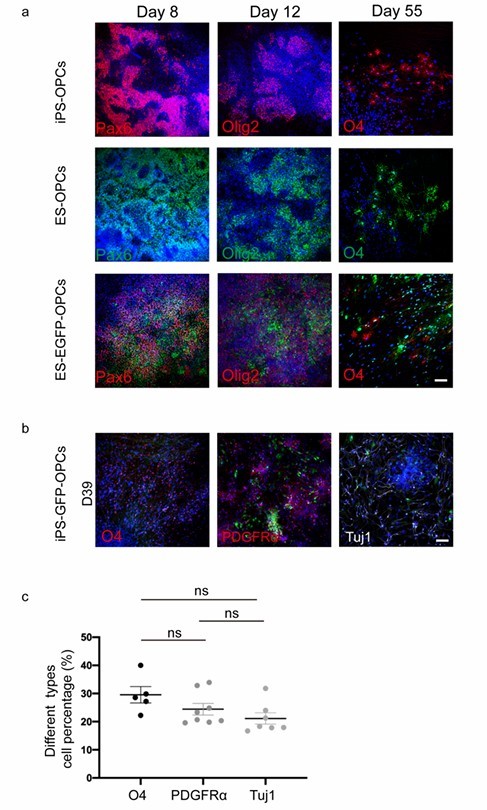 Fig. 2. Three cell lines were successfully induced to differentiate into OPCs (Li Q, Liu S, et al., 2024).
Fig. 2. Three cell lines were successfully induced to differentiate into OPCs (Li Q, Liu S, et al., 2024).
Puerarin Promotes Generation of Human iPSC-derived Pre-Oligodendrocytes
Myelin damage is common in neurological diseases like multiple sclerosis (MS), leading to impaired nerve function. Puerarin, a natural isoflavone, has shown neuroprotective effects through anti-inflammatory and antioxidant pathways. Xu's team evaluated puerarin's benefits on oligodendrocyte progenitor cells (OPCs) derived from human-induced pluripotent stem cells (hiPSCs), their differentiation processes.
To investigate puerarin's effect on OPC and OL differentiation from hiPSC-derived neural stem cells (NSC), they optimized a protocol (Douvaras & Fossati, 2015), starting from hiPSC to NSC and then adding reagents for differentiation into OPC and OL, as shown in Figure 3a. Over 55 to 75 days, hiPSCs were differentiated into Olig2+ OL lineage cells expressing O4 and MBP to varying degrees. The cell morphology at different stages is depicted in Figure 3b–g. Marker expression was monitored via immunocytochemistry; NSC markers like PAX6/SOX1/SOX2/Nestin appeared around Day 8 (Fig. 3h, i). By Day 30, cell aggregates expressing Olig2 and NKx2.2 indicated OL lineage commitment (Fig. 3j, k). Double-positive OPC markers Olig2 and NKX2.2 were identified in emigrating cells from aggregates by Days 35–40 (Fig. 3l). PreOL marker O4 and mature OL marker MBP were observed by Day 55 (Fig. 3m). The emergence of neurons and astrocytes accompanied the emigrating oligodendrocyte lineage cells. To assess how puerarin affects hiPSC-OPC differentiation into OLs, cells were treated with puerarin from Day 30 to Day 55 during OPC differentiation with oligodendrocyte factors (PDGF, NT3, T3, IGF-1, HGF), as depicted in Figure 3a. OL lineage markers in iPSC-derived cells were analyzed, with DMSO-treated cells as the control. By Day 55, Olig2+ NKx2.2+ OPC proportions were similar between puerarin and controls, but puerarin significantly increased O4+ cells (19.07% vs. 31.88%, Fig. 4c, d), confirmed by live staining on Days 65-67. These findings indicate puerarin enhances preOL generation.
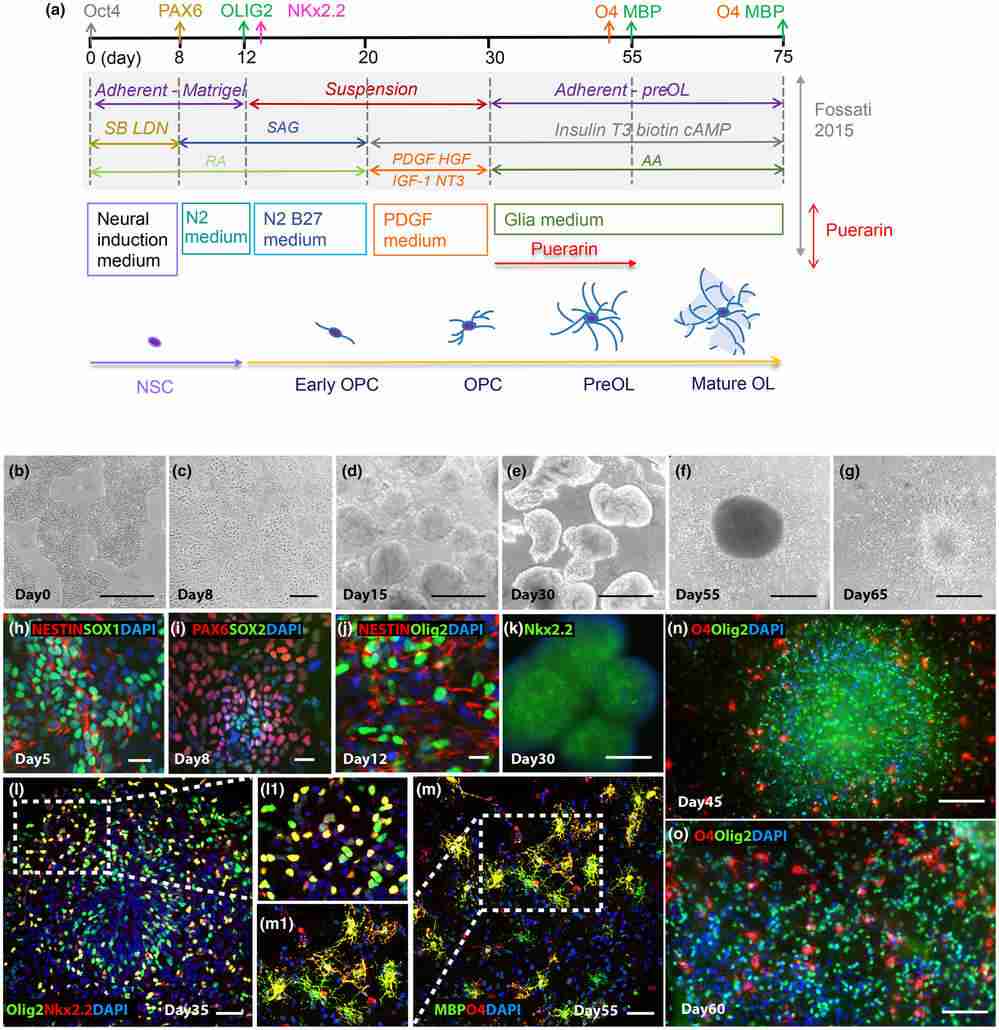 Fig. 3. Differentiation of human induced pluripotent stem cells (hiPSCs) into oligodendrocytes (OLs) through neural stem cells (NSCs) (Xu H, Zhang H, et al., 2024).
Fig. 3. Differentiation of human induced pluripotent stem cells (hiPSCs) into oligodendrocytes (OLs) through neural stem cells (NSCs) (Xu H, Zhang H, et al., 2024).
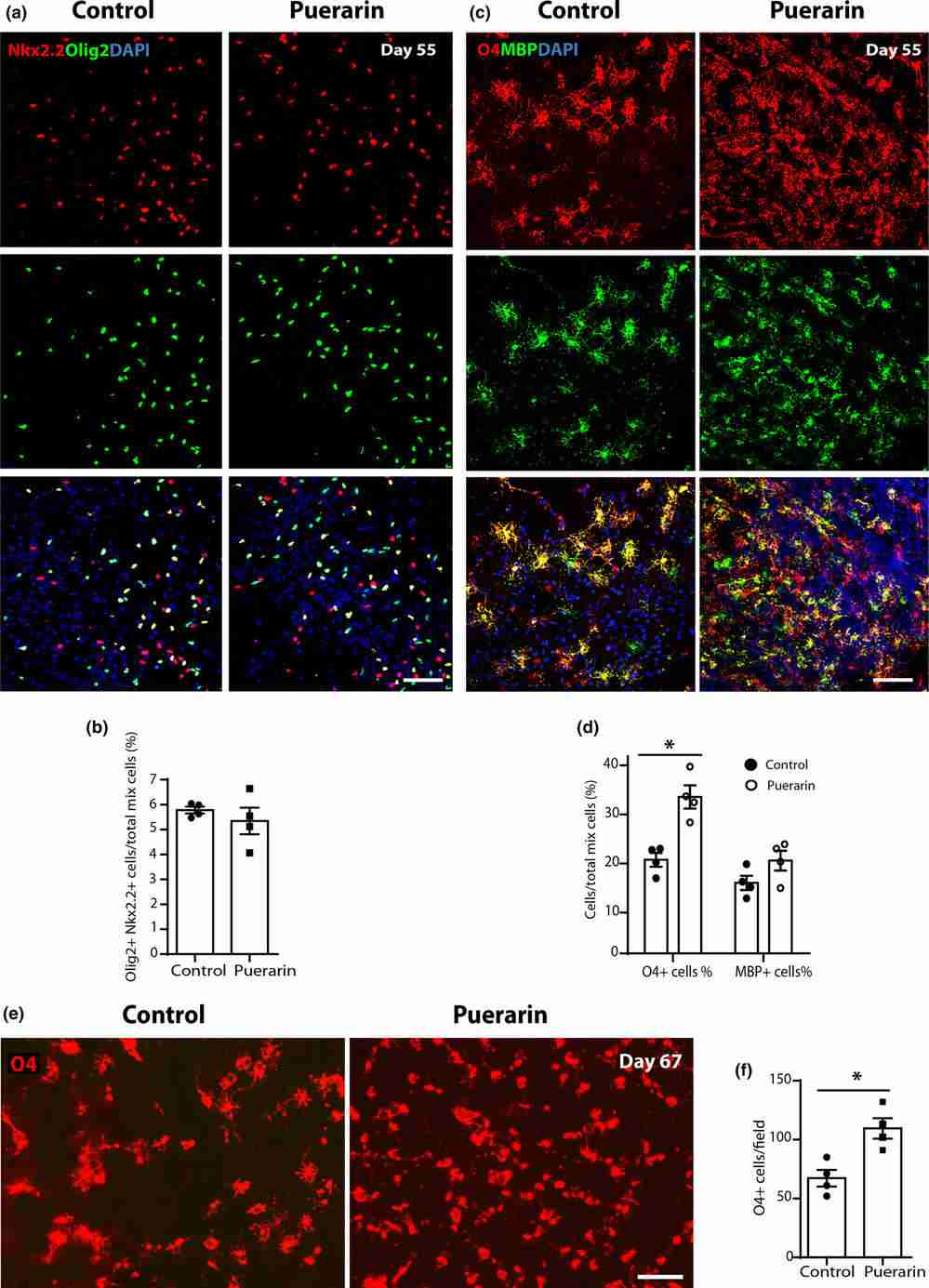 Fig. 4. Puerarin promotes generation of hiPSC-derived pre-oligodendrocytes (Xu H, Zhang H, et al., 2024).
Fig. 4. Puerarin promotes generation of hiPSC-derived pre-oligodendrocytes (Xu H, Zhang H, et al., 2024).
Ask a Question



