PEI MAX® - Transfection Grade Linear Polyethylenimine Hydrochloride (MW 40,000) [49553-93-7]
Référence 24765-100
Conditionnement : 100mg
Marque : Polysciences
PEI MAX® - Transfection Grade Linear Polyethylenimine Hydrochloride (MW 40,000)
| Catalog Number | Unit Size | QTY | |
|---|---|---|---|
| 24765 (100) | 100mg | | |
| 24765 (1) | 1g | | |
Product Overview
Polyethylenimine “Max” (PEI MAX) is a powerful, trusted, and cost-effective reagent widely considered as a current gold standard for both in vitro and in vivo transfection. PEI MAX has a high density of protonatable amino groups, with amino nitrogen as every third atom. This imparts a high buffering ability at nearly any pH. Hence, once inside the endosome, PEI MAX disrupts the vacuole and releases the genetic material into the cytoplasm. Stable complexation with DNA, efficient entry into the cell, and ability to escape the endosome makes PEI MAX a highly efficient transfection reagent which is compatible for a wide range of cell lines/types including the most commonly used HEK293 and CHO cells grown in adherent and suspension cultures. PEI MAX is capable of yielding high efficiency cell lines without compromising cell viability compared to other PEI and liposomal transfection reagents available in the market.
Key Advantages
- Superior Performance: High transfection efficiency with low cytotoxicity compared to other reagents on the market, suitable for use in larger concentrations and in sensitive cells.
- Low cytotoxicity: Even larger concentrations of PEI MAX disrupts cells minimally
- High Quality: Sourced and manufactured in the U.S. under ISO 13485 Quality System
- Flexible Workflow: Easy to optimize and introduce into application protocols. Scalable for well plates, flasks, and larger capacity bioreactors.
- Available in cGMP grade and in both powder and liquid formats.
- Cost-Effective: Economical compared to similar transfection products in the market.
- History: Polysciences is a trusted partner for pharma and medical device key players with more than 60 years in specialty chemical manufacturing. PEI MAX has been used by thousands of customers in their research and development for more than a decade.
POLYSCIENCES’ PEI MAX PRODUCT YIELDS HIGH TITERS OF AVV AFTER 72 HOURS POST TRANSFECTION
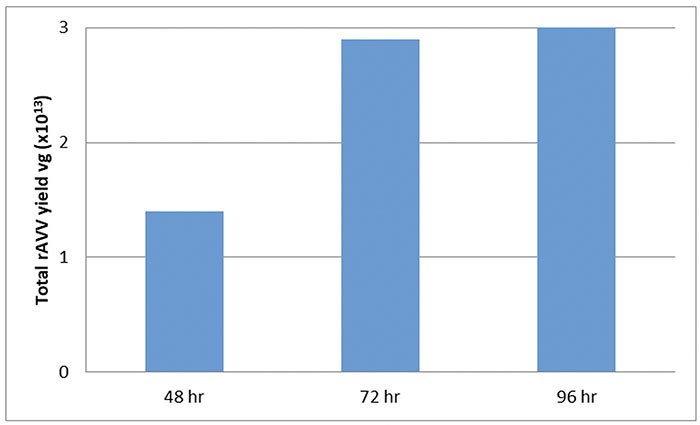
Flasks containing 30mL cultures of suspension HEK293 cells at 1×10^6 cells/mL were transfected to produce rAAV8 CMV-eGFP. rAAV8 was harvested from the media 48 hours post-transfection with increasing amounts of vector found in the media over time (Griger 2015).
Properties
Insoluble in: Common organic solvents (ethanol, acetone, tetrahydrofuran)
References
Baranyi, L., Doering, C. B., Denning, G., Gautney, R. E., Harris, K. T., Spencer, H. T., ... & Dropulic, B. (2013). Rapid generation of stable cell lines expressing high levels of erythropoietin, factor VIII, and an antihuman CD20 antibody using lentiviral vectors. Human Gene Therapy Methods, 24(4), 214-227.
Chen, S. H., Papaneri, A., Walker, M., Scappini, E., Keys, R. D., & Martin, N. P. (2020). A Simple, Two-Step, Small-Scale Purification of Recombinant Adeno-Associated Viruses. Journal of Virological Methods, 113863.
Cohen-Dvashi, H., Zehner, M., Ehrhardt, S., Katz, M., Elad, N., Klein, F., & Diskin, R. (2020). Structural Basis for a Convergent Immune Response against Ebola Virus. Cell Host & Microbe, 27(3), 418-427.
Costard, L. S., Kelly, D. C., Power, R. N., Hobbs, C., Jaskaniec, S., Nicolosi, V., ... & O’Brien, F. J. (2020). Layered Double Hydroxide as a Potent Non-viral Vector for Nucleic Acid Delivery Using Gene-Activated Scaffolds for Tissue Regeneration Applications. Pharmaceutics, 12(12), 1219.
Delafosse, L., Xu, P., & Durocher, Y. (2016). Comparative study of polyethylenimines for transient gene expression in mammalian HEK293 and CHO cells. Journal of Biotechnology, 227, 103-111.
Fernandez-Sendin, M., Tenesaca, S., Vasquez, M., Aranda, F., & Berraondo, P. (2020). Production and use of adeno-associated virus vectors as tools for cancer immunotherapy. In Methods in Enzymology (Vol. 635, pp. 185-203). Academic Press.
El Andari, J., & Grimm, D. (2020). Production, processing, and characterization of synthetic AAV gene therapy vectors. Biotechnology Journal, 2000025.
Gutiérrez-Granados, S., Cervera, L., de las Mercedes Segura, M., Wölfel, J., & Gòdia, F. (2016). Optimized production of HIV-1 virus-like particles by transient transfection in CAP-T cells. Applied microbiology and biotechnology, 100(9), 3935-3947.
Jia, N., Wang, J., Shi, W., Du, L., Sun, Y., Zhan, W., ... & Microbiome Consortium. (2020). Large-scale comparative analyses of tick genomes elucidate their genetic diversity and vector capacities. Cell, 182(5), 1328-1340.
Kobayashi, S., Yoshii, K., Hirano, M., Muto, M., & Kariwa, H. (2017). A novel reverse genetics system for production of infectious West Nile virus using homologous recombination in mammalian cells. Journal of virological methods, 240, 14-20.
Longo, P. A., Kavran, J. M., Kim, M. S., & Leahy, D. J. (2013). Transient mammalian cell transfection with polyethylenimine (PEI). In Methods in enzymology (Vol. 529, pp. 227-240). Academic Press.
Mann, J. F., McKay, P. F., Arokiasamy, S., Patel, R. K., Klein, K., & Shattock, R. J. (2013). Pulmonary delivery of DNA vaccine constructs using deacylated PEI elicits immune responses and protects against viral challenge infection. Journal of controlled release, 170(3), 452-459.
Pinnapireddy, S. R., Duse, L., Strehlow, B., Schäfer, J., & Bakowsky, U. (2017). Composite liposome-PEI/nucleic acid lipopolyplexes for safe and efficient gene delivery and gene knockdown. Colloids and Surfaces B: Biointerfaces, 158, 93-101.
Puente-Massaguer, E., Gòdia, F., & Lecina, M. (2020). Development of a non-viral platform for rapid virus-like particle production in Sf9 cells. Journal of Biotechnology, 322, 43-53.
Puente-Massaguer, E., Strobl, F., Grabherr, R., Striedner, G., Lecina, M., & Gòdia, F. (2020). PEI-mediated transient transfection of high five cells at bioreactor scale for HIV-1 VLP production. Nanomaterials, 10(8), 1580.
Schmit, P. F., Pacouret, S., Zinn, E., Telford, E., Nicolaou, F., Broucque, F., ... & Vandenberghe, L. H. (2020). Cross-Packaging and Capsid Mosaic Formation in Multiplexed AAV Libraries. Molecular Therapy-Methods & Clinical Development, 17, 107-121.
Song, L., Bower, J. J., & Hirsch, M. L. (2020). Preparation and administration of adeno-associated virus vectors for corneal gene delivery. In Corneal Regeneration (pp. 77-102). Humana, New York, NY.
Stuible, M., Burlacu, A., Perret, S., Brochu, D., Paul-Roc, B., Baardsnes, J., ... & Durocher, Y. (2018). Optimization of a high-cell-density polyethylenimine transfection method for rapid protein production in CHO-EBNA1 cells. Journal of biotechnology, 281, 39-47.
Thomas, M., Lu, J. J., Ge, Q., Zhang, C., Chen, J., & Klibanov, A. M. (2005). Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proceedings of the National Academy of Sciences, 102(16), 5679-5684.




