CD163 Antibody
Katalog-Nummer OASA01364
Size : 25ug
Marke : Aviva Systems Biology
| Datasheets/Manuals | Printable datasheet for CD163 Antibody (OASA01364) |
|---|
| Predicted Species Reactivity | Human |
|---|---|
| Product Format | Liquid. Phosphate buffered saline |
| Clonality | Monoclonal |
| Clone | EDHu-1 |
| Isotype | IgG1 |
| Host | Mouse |
| Application | ELISA, FC, ICC, IF, IHC-Fr, IHC-P, WB |
| Reconstitution and Storage | -20°C |
| Immunogen | Leucocytes harvested from the pleural cavity of patients with idiopathic spontaneous pneumothorax |
| Purification | Protein A Purified |
| Concentration | 1 mg/ml |
| Specificity | Mouse anti Human CD163 antibody, clone EDHu-1 recognizes the human CD163 cell surface antigen, a 130-140 kDa glycoprotein expressed by tissue macrophages. CD163 is not expressed by resting peripheral blood leucocytes, but expression may be induced on monocytes by culture in dexamethasone. Clone EDHu-1 is reported to inhibit the binding of haptoglobin/hemoglobin to CD163 (Madsen et al. 2004). Truncation mutation analysis demonstrates binding of EDHu-1 occurs via the N-terminal region of CD163 containing the first three scavenger receptor, Cysteine-rich, SRCR domains the third domain being critical as, cleavage of this domain at the major cleavage site ASP-265 abrogates binding to the N-terminal fragment. |
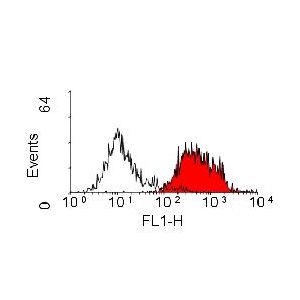
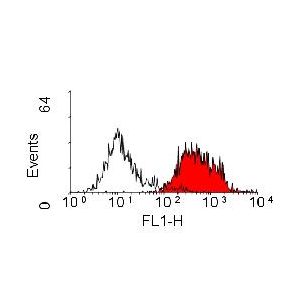
| Application Info | Flow Cytometry: Use 10ul of the suggested working dilution to label 106 cells in 100ul. |
| Protocol Information | Citation: 1: Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S, Panina-Bordignon P, Manfredi AA, Rovere-Querini P. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. 2009 Aug;175(2):547-56. Epub 2009 Jul 2. PubMed PMID: 19574425; PubMed Central PMCID: PMC2716955. Species: Human, Mice Experiment Name: 1. In Vivo Macrophage Depletion2. Macrophages Assessment in the Mouse Peritoneal Liquid3. Immunohistochemistry of Murine Lesions Experiment Background: 1. Macrophages in endometriotic lesions, leiomyomas, and apparently nonaffected peritoneum from patients with endometriosis and controls were characterized by immunohistochemistry for the expression of a lineage marker (CD68) and for markers of alternative activation (CD163 and CD206).2. In this study, using an experimental mouse model, Monica et al. verified the hypothesis that macrophage activation is involved in the establishment, survival, and spreading of ectopic lesions from shed endometrium. Monica et al. finely characterized macrophages in the peritoneal fluid and in established endometriotic lesions, in experimental mice and human, and found that they share evident features of alternative, disease-permissive, activation. Experimental Steps: 1. In Vivo Macrophage DepletionTo verify the role of macrophages in the establishment and growth of ectopic endometrial lesions, mice were treated i.p. with the depleting anti-F4/80 Ab (0.80 ug/g/ mice clone CI: A3-1, , Oxford, UK) or PBS, or with liposomes containing either clodronate (Sigma Aldrich, St Louis, MO) or PBS. When indicated, depleting agents were injected intraperitoneally. The anti-F4/80 depleting mAb was injected at day -2, the treatment was repeated at day 0, +2, +4, +6, +8, +10 after uterus transfer. Liposomes containing PBS or clodronate were injected at days -1, 0, +4, and +8 after endometrial tissue transfer. The final clodronate liposome suspension contained 5 mg of clodronate/ml. Both treatments kill monocytes and macrophages, while are not per se otherwise toxic. Control mice were injected i.p. with PBS. To assess the effect of macrophage depletion on ectopic endometrial tissue early survival and adhesion to the peritoneal cavity, depletion was performed only at day 0. To assess the effect of macrophage depletion on already established lesions, the treatment was performed exclusively at days +4, +8, ie, after lesion engraftment. To verify the efficacy of the treatment, blood or peritoneal fluid was retrieved at various times and analyzed by flow cytometry. Samples (30 ul) were incubated for 10 minutes at room temperature with PBS containing 10% fetal calf serum. Phycoerythrin-conjugated anti-F4/80 mAb (clone CI: A3-1, ) and/or allophycocyanin-conjugated anti-CD11b mAb (clone M1/70, BD Biosciences) (2 ul/sample) were added for 20 minutes. Red blood cell lysis buffer (0.15 M/L NH4Cl, 1 mmol/L KHCO3, and 0.1 mmol/L Na2EDTA) was added for 10 minutes at room temperature before analysis (FACS Calibur flow cytometer and CellQuest software, BD Biosciences).2. Macrophages Assessment in the Mouse Peritoneal LiquidPeritoneal cells were retrieved by peritoneal lavage of treated and untreated animals, and resuspended in cold PBS containing 2 mmol/L EDTA. Cell viability, verified by trypan blue exclusion, was typically >98%. Cells were stained with allophycocyanin-conjugated anti-CD11b Ab (BD, Pharmingen), phycoerythrin-conjugated anti-F4-80 Ab (R&D), or with purified unlabeled rat anti-mouse anti-CD206 Ab and purified antibody rabbit anti-mouse anti-CD163. Phycoerythrin-conjugated goat anti-rat or fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit Ab were used as second step reagents before analysis by 2- and 4-parametric flow cytometry.3. Immunohistochemistry of Murine LesionsEndometriotic lesions were frozen in liquid N2-cooled isopentane. Serial 6-mm thick sections were fixed with 4% paraformaldehyde (10 minutes at room temperature) and successively treated with 0.3% H2O2 (10 minutes at room temperature) to quench endogenous peroxidase activity. To evaluate macrophage infiltration, tissue sections were incubated in PBS + 5% bovine serum albumin for 1 hour at room temperature and then overnight at 4C with rat anti-mouse CD68 mAb (2 mg/ml, clone FA-11, ), CD163 (1 mg/ml, clone M-96, Santa Cruz Biotechnology, Inc, Santa Cruz, CA), and CD206 (1 mg/ml, clone MR5D3, ). Endothelial cells were identified by staining with anti-mouse CD31 mAb. Primary Abs were revealed using biotin conjugated anti-rat polyclonal IgG (1.5 mg/ml, eBiosciences, San Diego, CA) and R.T.U horseradish peroxidase streptavidin (Vector Laboratories, Burlingame, CA), which was detected using Vector NovaRED substrate kit (Vector Laboratories). Slides were counterstained with hematoxylin and examined under a Nikon Eclipse 55i microscope (Nikon, Tokyo, Japan). Images were captured with Digital Sight DS-5 M digital camera (Nikon) using Lucia G software (Laboratory Imaging, Prague, CZ). Parallel slides in which primary Abs had been omitted were identically processed and used as negative controls. Number Of Protocols: 3 |
| Reference | 1. Kristiansen, M. et al. (2001) Identification of the haemoglobin scavenger receptor. Nature 409: 198-201. 2. Madsen, M. et al. (2004) Molecular characterization of the haptoglobin.hemoglobin receptor CD163. Ligand binding properties of the scavenger receptor cysteine-rich domain region. J. Biol. Chem. 279: 51561-51567. 3. Kim, W.K. et al. (2006) CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am. J. Pathol. 168: 822-834. 4. Moreno, J.A. et al. (2010) Peripheral Artery Disease Is Associated With a High CD163/TWEAK Plasma Ratio. |
|---|---|
| Gene Symbol | CD163 |
| Alias Symbols | M130, MM130, SCARI1 |
| NCBI Gene Id | 9332 |
| Protein Name | Scavenger receptor cysteine-rich type 1 protein M130 |
| Description of Target | MOUSE ANTI HUMAN CD163 |
| Uniprot ID | Q86VB7 |
| Protein Accession # | NP_004235.3 |
| Protein Size (# AA) | 1156 |