SBE-β-CD (Sulfobutylether beta-cyclodextrin)
Cat# M4837-5g
Size : 5g
Brand : AbMole Bioscience
All AbMole products are for research use only, cannot be used for human consumption.

Sulfobutylether-β-Cyclodextrin
Quality Control & Documentation
Biological Activity
SBE-β-CD (Sulfobutylether beta-cyclodextrin) is a unique reproducible mixture of polyanionic β-cyclodextrin derivatives in which a sodium sulfonate salt is tethered to the lipophilic cyclodextrin cavity by a butyl ether group, or sulfobutylether (SBE). The sulfobutyl ether (SBE) substituent is introduced at the 2, 3, and 6 positions in one or more of the glucopyranose units in the cyclodextrin structure.
Product Citations
-

Cell Rep. 2023 Jun 27;42(7):112690.
Identification of XAF1 as an endogenous AKT inhibitor
SBE-β-CD (Sulfobutylether beta-cyclodextrin) purchased from AbMole -

Int J Mol Sci. 2023 Feb 6;24(4):3229.
The Role of the Dopamine System in Post-Stroke Mood Disorders in Newborn Rats
SBE-β-CD (Sulfobutylether beta-cyclodextrin) purchased from AbMole -

The American Journal of Pathology. 2022 Dec.
EZH2 Promotes Cholangiocarcinoma Development and Progression through Histone Methylation and microRNA-Mediated Down-Regulation of Tumor Suppressor Genes
SBE-β-CD (Sulfobutylether beta-cyclodextrin) purchased from AbMole -

Anal Cell Pathol (Amst). 2022 Oct 5;2022:3770715.
Chemoprevention of 4NQO-Induced Mouse Tongue Carcinogenesis by AKT Inhibitor through the MMP-9/RhoC Signaling Pathway and Autophagy
SBE-β-CD (Sulfobutylether beta-cyclodextrin) purchased from AbMole -

Int J Cancer. 2013 Nov;133(9):2065-76.
Combined targeting of AKT and mTOR using MK-2206 and RAD001 is synergistic in the treatment of cholangiocarcinoma.
SBE-β-CD (Sulfobutylether beta-cyclodextrin) purchased from AbMole
Customer Product Validations & Biological Datas
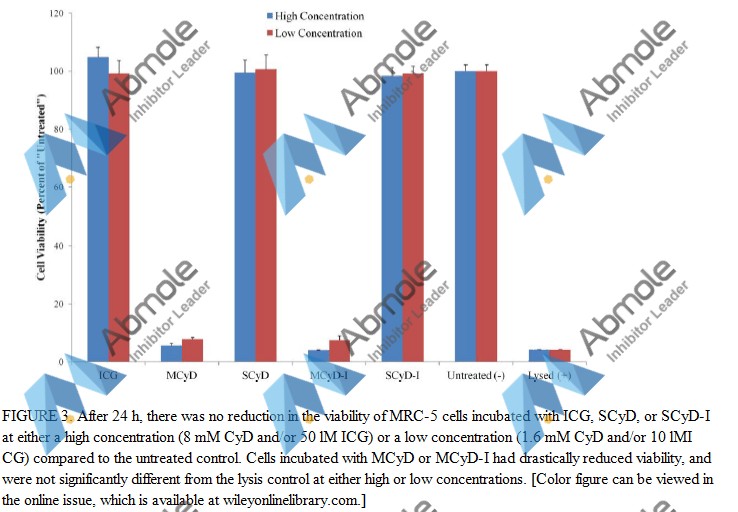 | Source | J Biomed Mater Res B Appl Biomater (2016). Figure 3. Captisol |
| Method | Transmission electron microscopy | |
| Cell Lines | MRC-5 cells | |
| Concentrations | 1 μM | |
| Incubation Time | 24 h | |
| Results | Deionized water-dispersed ICG, SCyD, and MCyD showed a size distribution ranging from 2 to 200 nm. |
 | Source | J Biomed Mater Res B Appl Biomater (2016). Figure 1. Captisol |
| Method | Fluorescence | |
| Cell Lines | ||
| Concentrations | 10 mM | |
| Incubation Time | 24 h | |
| Results | Over 24 h, ICG retained just 0.383 of its initial fluorescence in water, while MCyD-I and SCyD-I in water retained 1.943 and 2.063 higher fluorescence than ICG at t0. In PBS, ICG retained 0.753 of its initial fluorescence while MCyD-I actually increased to 29.863 and SCyD-I retained 20.833 of initial ICG fluorescence in PBS. |
Protocol (for reference only)
| Cell Experiment | |
|---|---|
| Cell lines | Caco-2 cells |
| Preparation method | The cells were then preincubated for 30 min without Nos solution+permeation enhancer CH or CP and then incubated for 120 min at 37 ℃ with the Nos solution (50 mg/ml)+permeation enhancer CH or CP. |
| Concentrations | 50 mg/ml |
| Incubation time | 2 h |
| Animal Experiment | |
|---|---|
| Animal models | pig |
| Formulation | 10-mL solution composed of 25% water and 75% PEG400 |
| Dosages | 5 mg/kg |
| Administration | bolus infusions |
Chemical Information
| Molecular Weight | 1451.29 |
| Formula | C50H84Na2O41S2 |
| CAS Number | 182410-00-0 |
| Solubility (25°C) | Water > 120 mg/mL |
| Storage | Powder -20°C 3 years ; 4°C 2 years In solvent -80°C 6 months ; -20°C 1 month |
References
[1] Mamoru Fukuda, et al. Int J Pharm. Influence of sulfobutyl ether beta-cyclodextrin (Captisol) on the dissolution properties of a poorly soluble drug from extrudates prepared by hot-melt extrusion
[2] Valentino J Stella, et al. Sulfobutylether-β-cyclodextrin
[3] Farouk Semcheddine, et al. Effects of the Preparation Method on the Formation of True Nimodipine SBE-β-CD/HP-β-CD Inclusion Complexes and Their Dissolution Rates Enhancement
[4] David R Luke, et al. Review of the basic and clinical pharmacology of sulfobutylether-beta-cyclodextrin (SBECD)
[5] Valentino J Stella, et al. Cyclodextrins
[6] V Zia, et al. Effect of cyclodextrin charge on complexation of neutral and charged substrates: comparison of (SBE)7M-beta-CD to HP-beta-CD



