Immunohistochemistry protocol - Avidin/Biotin Method (ABC)
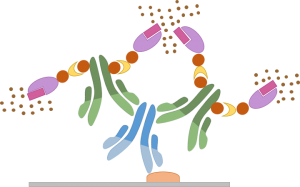
The principle of ABC detection kits is based on the affinity of avidin (or streptavidin) for biotin. Indeed, abbreviation ABC stands for Avidin / Biotin Complex.
In general, the ABC detection kits are composed of:
- A biotinylated secondary antibody
- An Avidin / Enzyme Complex
The kits may or may not contain the blocking and dilution buffers and the substrate depending on the formats.
I. Required material
Reagents
- A primary antibody , labeled or not, against the target molecule
- Revelation kit
- Chromogen
Buffers
- Xylene
- Alcooholic baths at 70%, 80%and 95%
- Hydrogen peroxide (H2O2)
- Tris EDTA, Tris HCl
- Tween-20
Reagents
- A primary antibody , labeled or not, against the target molecule
- Revelation kit
- Chromogen
Buffers
- Xylene
- Alcooholic baths at 70%, 80%and 95%
- Hydrogen peroxide (H2O2)
- Tris EDTA, Tris HCl
- Tween-20
II. Experimentation time
- 10 minfor peroxydase blocking
- 30 minfor antigen retrieval (if necessary)
- 30-60 minfor incubation with primary antibody
- 10 minfor inubation with biotinylated secondary antibody
- 10 minfor incubation with Streptvidin peroxidase conjugate
- 5 minfor chromogen
- 5 minfor chromogen
- Hematoxylin staining (optional)
- TOTAL : 2-3 hours
- TOTAL : 2-3 hours
III. Protocol
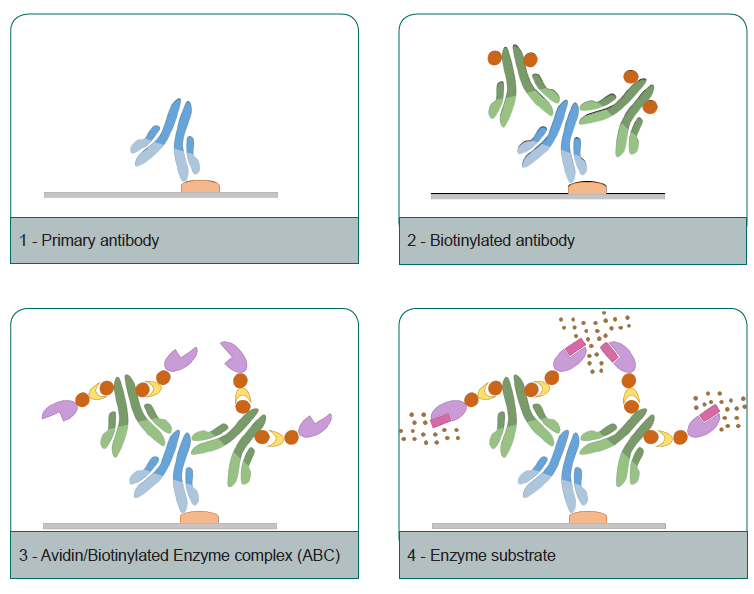
- Peroxydase blockin : Apply 2 drops (100 µl) or enough volume of peroxydase blocking reagent (3%H2O2 solution) to cover the tissue section and incubate. Rinse the slide using distilled water.
- HIER pretreatment (refer to antibody specification sheet) : Heat Induced Epitope Retrieval (HIER) may be required for primary antibody suggested by vendor. Wash with PBS 2 min, 3 times.
- Pre-blocking : Add 2 drops or enough volume of Pre-blocking solution to completely cover the tissue sectin and incubate. Blot off solution, Do not rinse.
- Primary antibody (supplied by user) : Apply 2 drops or enough volume of Primary antibody to cover the tissue section completely. Incubate in moist chamber for 30-60 min.
- Secondary antibody : Apply 2 drops or enough volume of Primary antibody to cover the tissue section completely. Incubate for 10 min.
- HRP-Streptavidin : Apply 2 drops or enough volume of HRP-Streptavidin to cover the tissue section completely and incubate 10 min. Rinse with PBS for 2 min, 3 times.
- Chromogen : Add 1 drop or 2 drops (for higher sensitivity and contrast) of chromogen concentrate to 1 ml of Substrat. Mix weel, protect from light and use within 5 hours. Aplly 2 drops (100 µl) or enough volume of premixed chromogen to completely cover tissue and incubate 5 minutes. Rinse with distilled water for 2 min, 3 times.
- Hematoxylin (optional) : Counterstain with 2 drops (100 µl) or more drops to cover tissue completely and wait about 10-20 sec. Rinse thoroughly under tap water for 1-2 min. Put slides in PBS until show blue color (about 30-60 sec). Rinse well in distilled watr.
- Mounting : Follow the manufacturer data sheet procedurefor mounting.
Notes :
- The fixation, tissue slide thickness, antigen retrieval and primary dilution and incubation time effect results significantly. Investigator needs to consider all factors and determine optimal conditions when interpret the result.
- Tissue staining is dependent upon the proper handling and processing of tissues prior to staining. Improper tissue preparation may lead to false negative results or inconscient results.
- Do not mix reagents from different lots.
- Do not allow the slides to dry at any time during staining.

