Copner Biotech’s 24-Well, 3D PETG Cell Culture Scaffolds
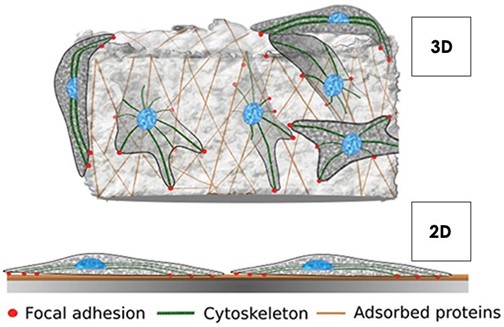
2D v 3D Cell Culture
The benefits of 3D cell culture over conventional 2D culture are widely accepted in the scientific community.
Traditional 2D cell culture methods subject cells to an un-physiological architectural state. When cultured in 2D, mammalian cells assume a bipolar state with a basal and apical side. In order to tackle this unnatural morphology, cytoskeletal re-modelling takes place and the subsequent ultrastructure of the cell is greatly altered (Ravi et al., 2015). Cells cultured in 2D also have unlimited access to oxygen, nutrients and metabolites –which is not the case in their respective tissues in vivo. These limitations ultimately mean those laboratories undertaking assays on cells in this unnatural environment yield less reliable results in vitro.
3D cell culture creates a 3D architecture for cell growth, offering a closer knit and physiologically relevant environment. Growth of cells on 3D scaffolds promotes accurate cell-to-cell and cell-to-extracellular environment interactions with variable access to oxygen, nutrients and metabolites. Mammalian cells that sit within a more representative environment, reflective of their host tissue in vivo, exhibit more relevant gene expression, splicing topology and biomarkers in vitro, which in turn offers far greater reliability in terms of drug and toxicity modelling in the lab (Lv et al., 2016).
Scaffold Design
Recent studies demonstrate the importance of discrete oxygen gradients in cell movement across the scaffold interface (Ardakani et al, 2014). Cells grown on woodpile structures typically have a heterogenous distribution, with clusters common. By introducing a discrete oxygen gradient across the interface of our 3D PETG scaffold, we encourage cell proliferation from the centre to the periphery. The result is a much more balanced system of cells on the scaffold, with confluency patterns like that of in vivo tissue.
Copner Biotech have developed a bespoke 3D modelling software and next generation 3D printing technique in order to create these complex architectures. Our additive manufacturing process demonstrates high batch-to- batch consistency, minimising variables in your 3D cell models.
|
|
|
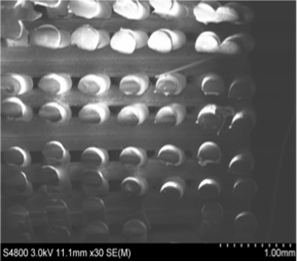
Scaffold microstructure validation work - (Left) Top view of PETG scaffold using a standard light microscope. (Right) Cross section of a PETG scaffold captured using scanning electron microscopy (SEM).
Sterilisation and Cell Seeding Protocols
Notes Before Starting
- Prior to use, scaffolds must be rendered hydrophilic with a 70 % ethanol wash for 15 minutes. Please note, scaffolds must be placed in the correct orientation prior to use (see figure below). When washing with ethanol, be sure to pipette solution thoroughly through the scaffold’s interconnected pores.
- Add enough ethanol to completely cover the scaffold, remove, and wash 2x with appropriate culture media (2ml).
- To avoid drying, leave the scaffold in the final medium (2ml) wash until required.
- We recommend leaving scaffolds in this final medium for 4h to enhance subsequent cell attachment.

Correct Scaffold Orientation – The scaffold to the far left represents the correct orientation for use, whilst the other scaffolds are upside down. Please note that the base of the scaffolds can vary in appearance.
Seeding Cells on Scaffold
- Remove all cell culture media from the well prior to seeding, via pipette.
- Slowly pipette your desired cell density via a 100ul cell media solution onto the top of the scaffold, taking care to ensure the solution permeates the interconnected pores as much as possible.
- Place seeded scaffolds back into the incubator for 3h to allow cell attachment.
- Following the 3h incubation, fill the remaining well with 1.4ml of complete medium.
- Change culture medium every 2 days until the end of culture.
- Cells can be recovered from the scaffold following a 1X trypsin treatment for 15 minutes.
- We recommend using several cell densities initially to optimise culture protocol prior to your experiments.
- Recommended cell density range 150,000 – 500,000 cells per scaffold.
Cellular Attachment
Copner Biotech’s 3D PETG scaffolds efficiently mediates mammalian cell attachment, whilst using low cell seeding concentrations. Cells used to date include, but are not limited to; L929 Fibroblasts, Keratinocytes, HeLa cells, MCF- 7 cells, A549 cells and iPSCs.
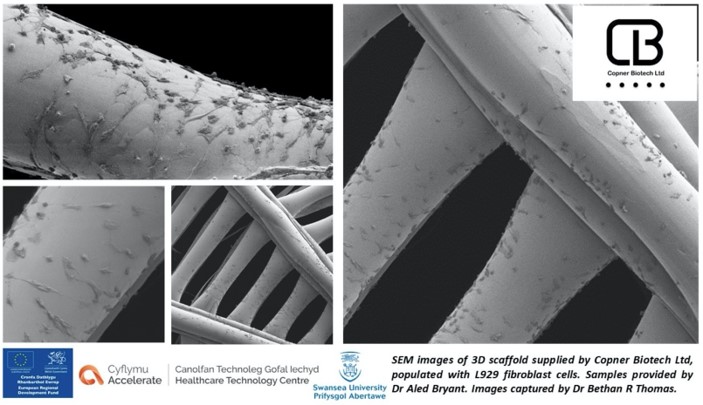
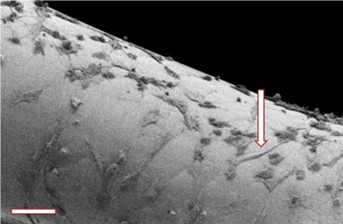
Scanning Electron Microscopy (SEM) imagesof L929 cells grown on PETG scaffold at day 7 (50K cells seeded at day 1). Cells demonstrate a successful migration from the centre to the periphery of the scaffold, creating a balanced, confluent cell system in the process.
Our bespoke 3D printing operating system, coupled with high precision printing technology, allows for the creation of specific scaffold regions with optimal surface roughness. These regions give rise to the first successfully attached cells from day 1 seeding and act as the start point for cell proliferation and migration to the periphery. Migration is stimulated by discrete oxygen and nutrient gradients across the scaffold interface, formed by the heterogeneity in pore size and distribution.
Cellular Viability
Our PETG material has been altered in order to optimise surface roughness and subsequently improve cell attachment and proliferation. This final inert, noncytotoxic material has been confirmed safe for use in cell culture, showing no negative effects on cell growth or function, making your transition from 2D to 3D cell culture easier.
3D PETG scaffolds also show rigidity and do not degrade, making them very user- friendly for long term culture of cells.
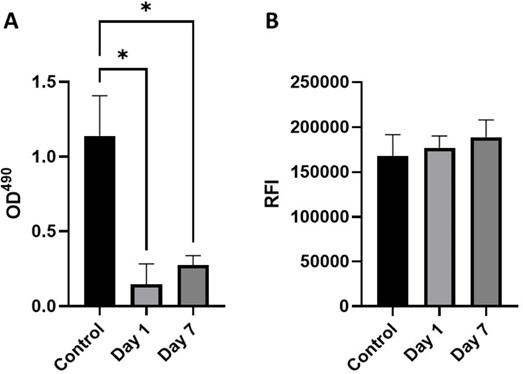
Cytotoxicity (A) LDH release (n=3) noted by optical density at 490 nm (B) Relative fluorescence intensity of alamarBlue assay (n=3). L929 cell line at a density of 1 x 104 cells were cultured with control media, day 1 conditioned media or day 7 conditioned media for 24h at 37°C. Figure 13. shows (A) LDH release (n=3) to each treatment condition as noted by optical density at 490 nm (B) Relative fluorescence intensity of almarBlue assay (n=3) to each treatment condition. Statistical significance determined by Friedmans test; * p < 0.05.
Cellular Proliferation
Our PETG material mediates cell division events, whilst minimising cell loss, resulting in significant expansion of cultures over a short space of time. This is particularly useful for those looking to study cells in the exponential growth phase or investigating cell-division events in real-time.
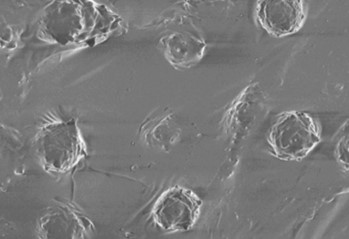
L929 cells below demonstrated a markedly increased ability to form elongated focal adhesion points on 3D PETG scaffolds, when compared to standard 2D culture. The focal adhesion to the various surfaces of the scaffold is important for the initiation of various signals, further stimulating cell proliferation and differentiation (Lee et al, 2004).
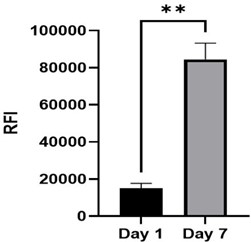 |
|
(Left) Relative fluorescence intensity of alamarBlue assay (n=3) at time points; day 1 and day
7. Statistical significance determined by paired t-test; * p < 0.05, ** p < 0.01. (Right) Elongated focal adhesion points, indicating a healthy fibroblast morphology in 3D culture.
Spheroid Formation
When using high cell seeding concentrations and culturing over a prolonged period (7 days+), 3D PETG scaffolds encourage the formation of spheroid cultures. Spheroid culture systems provide a similar physicochemical environment in vivo by facilitating cell-to-cell and cell-to-matrix interactions to overcome the limitations of traditional 2D cultures.
The 3D PETG scaffold system has interconnected pores throughout the entire structure and is designed to culture and harvest spheroid cultures with ease of use. By utilising the centre hole of the scaffold, users can aspirate and harvest spheroid cultures by pipetting up and down with minimal force. This novel method of spheroid culture and harvest allows users to create reliable 3D spheroid models, without using multiple steps and introducing human error into spheroid handling.
|
|
|
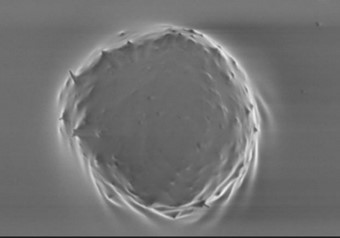
SEM images of dermal spheroids cultured on 3D PETG Scaffold. Fibroblasts and keratinocytes were cultured for 7 days, after seeding at 100K concentration on day 1. Spheroids were easily harvested from scaffolds by aspirating with pipette and showed reduced damage compared to methods of harvesting from hydrogel systems.
Organoid Formation
Further to spheroid formation, when used in conjunction with the appropriate growth factors, the 3D scaffolds can be used to generate early embryoid bodies, and subsequently organoids. Scaffolds can be used in long term culture of organoids, owing to their robust structure and strength.
|
|
|
|
|
|
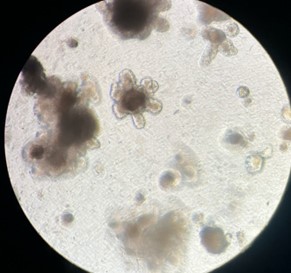
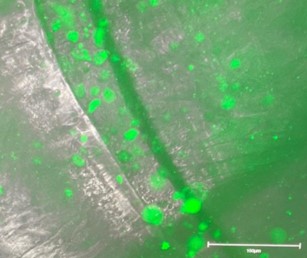
| Figure - Early embryoid body structures (top left image) can be pipetted from the scaffold structure and further cultured to form larger, complex organoid structures (top right image) in low attachment plates. Cellular structures can be stained and visualised via microscopy during and after culture (bottom right image). | |
| Product Details |