ubiquitin specific protease 3 fragment
Referencia A1097-25mg
embalaje : 25mg
Marca : APExBIO Technology
ubiquitin specific protease 3 fragment
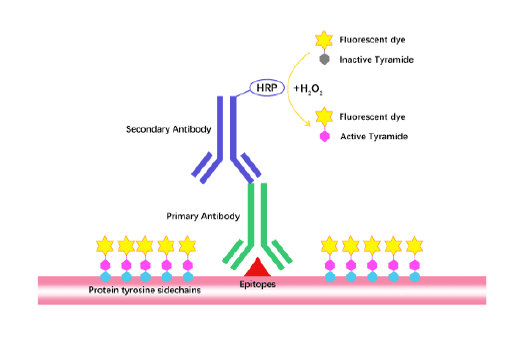
Tyramide Signal Amplification (TSA)
TSA (Tyramide Signal Amplification), used for signal amplification of ISH, IHC and IC etc.
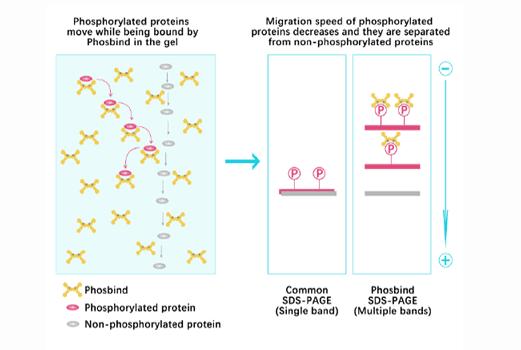
Phos Binding Reagent Acrylamide
Separation of phosphorylated and non-phosphorylated proteins without phospho-specific antibody
The ubiquitin specific protease 3 USP3 is a deubiquitinating enzyme for uH2A and uH2B. USP3 dynamically associates with chromatin and deubiquitinates H2A/H2B in vivo. The ZnF-UBP domain of USP3 mediates uH2AUSP3 interaction. Functional ablation of USP3 by RNAi leads to delay of S phase progression and to accumulation of DNA breaks, with ensuing activation of DNA damage checkpoint pathways. In response to ionizing radiation, (1) uH2A redistributes and colocalizes in g-H2AX DNA repair foci and (2) USP3 is required for full deubiquitination of ubiquitin-conjugates/uH2A and g-H2AX dephosphorylation. USP3 is a novel regulator of H2A and H2B ubiquitination, highlight its role in preventing replication stress, and suggest its involvement in the response to DNA double-strand breaks1.
USP3 has been characterized as a functional DUB in vitro, and it is the human DUB most homologous to S. cerevisiae Ubp8, which regulates H2B deubiquitination2-4.
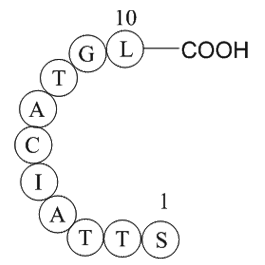
Figure1. Formula of Ubiquitin specific protease 3 fragment
Ref:
1.F. Nicassio, N. Corrado et al. Human USP3 Is a Chromatin Modifier Required for S Phase Progression and Genome Stability. Current Biology 17, 1972–1977.
2.Sloper-Mould, K.E., Eyre, H.J., Wang, X.W., Sutherland, G.R., and Baker, R.T. (1999). Characterization and chromosomal localization of USP3, a novel human ubiquitin-specific protease. J. Biol. Chem. 274, 26878–26884.
3.Henry, K.W., Wyce, A., Lo, W.S., Duggan, L.J., Emre, N.C., Kao, C.F., Pillus, L., Shilatifard, A., Osley, M.A., and Berger, S.L. (2003). Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8. Genes Dev. 17, 2648–2663.
4.Zhang, Y. (2003). Transcriptional regulation by histone ubiquitination and deubiquitination. Genes Dev. 17, 2733–2740.
| Physical Appearance | A solid |
| Storage | Store at -20°C |
| M.Wt | 937.07 |
| Formula | C38H68N10O15S |
| Synonyms | H2N-Ser-Thr-Thr-Ala-Ile-Cys-Ala-Thr-Gly-Leu-OH |
| Solubility | ≥93.7 mg/mL in DMSO; insoluble in H2O; insoluble in EtOH |
| SDF | Download SDF |
| Canonical SMILES | NC(CO)C(NC(C(O)C)C(NC(C(C)O)C(NC(C)C(NC(C(C)CC)C(NC(CS)C(NC(C)C(NC(C(O)C)C(NCC(NC(CC(C)C)C(O)=O)=O)=O)=O)=O)=O)=O)=O)=O)=O |
| Shipping Condition | Small Molecules with Blue Ice, Modified Nucleotides with Dry Ice. |
| General tips | We do not recommend long-term storage for the solution, please use it up soon. |