Hsp90 Antibody
Referentie OASE00019
Formaat : 50ug
Merk : Aviva Systems Biology
| Datasheets/Manuals | Printable datasheet for OASE00019 |
|---|
| Predicted Species Reactivity | Human, Mouse, Rat, Rabbit, Chicken, Plant, Fungi, Insect |
|---|---|
| Clonality | Monoclonal |
| Clone | AC-16 |
| Isotype | IgG2b |
| Host | Mouse |
| Conjugation | Unconjugated |
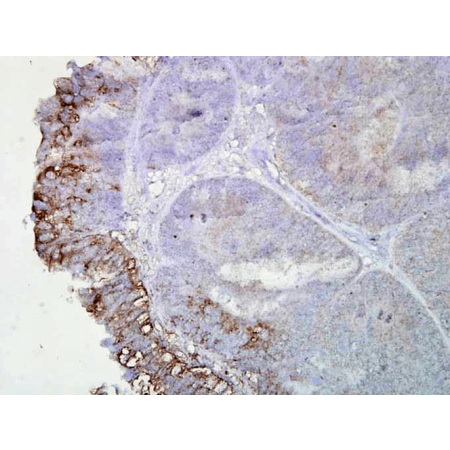
| Application | WB, IHC, AM |
| Additional Information | Background Info: This antibody is reactive with both the constitutive and the inducible form of Hsp90. It does not bind to the native form and does not recognize Hsp90 from E.coli or yeast. |
| :: | Scientific Background: Hsp90 is a highly conserved and essential stress protein that is expressed in all eukaryotic cells. From a functional perspective, hsp90 participates in the folding, assembly, maturation, and stabilization of specific proteins as an integral component of a chaperone complex (1-4). Despite its label of being a heat-shock protein, hsp90 is one of the most highly expressed proteins in unstressed cells (1–2% of cytosolic protein). It carries out a number of housekeeping functions – including controlling the activity, turnover, and trafficking of a variety of proteins. Most of the hsp90-regulated proteins that have been discovered to date are involved in cell signaling (5-6). The number of proteins now know to interact with Hsp90 is about 100. Target proteins include the kinases v-Src, Wee1, and c-Raf, transcriptional regulators such as p53 and steroid receptors, and the polymerases of the hepatitis B virus and telomerase.5 When bound to ATP, Hsp90 interacts with co-chaperones Cdc37, p23, and an assortment of immunophilin-like proteins, forming a complex that stabilizes and protects target proteins from proteasomal degradation. In most cases, hsp90-interacting proteins have been shown to co-precipitate with hsp90 when carrying out immuno-adsorption studies, and to exist in cytosolic heterocomplexes with it. In a number of cases, variations in hsp90 expression or hsp90 mutation has been shown to degrade signaling function via the protein or to impair a specific function of the protein (such as steroid binding, kinase activity) in vivo. Ansamycin antibiotics, such as geldanamycin and radicicol, inhibit hsp90 function (7). |
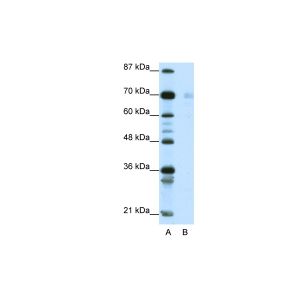
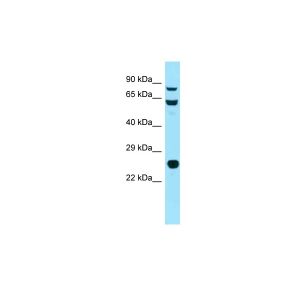
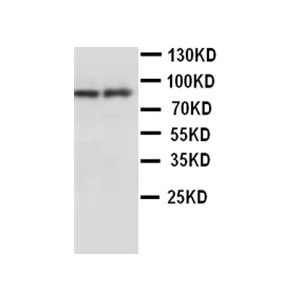
| :: | Certificate of Analysis: 1 ug/mL was sufficient for detection of Hsp90 in 20ug of heat shocked HeLa cell lysate by colorimetric immunoblot analysis using Goat anti-mouse IgG:HRP as the secondary antibody |
| Reconstitution and Storage | Store at -20C. Shipping with Blue Ice or 4C. |
| Immunogen | Heat shock protein 90 from the water mold Achyla ambisexualis |
| Purification | Protein G Purified |
| Concentration | 1 mg/ml |
| Specificity | Detects 90kDa. This antibody is reactive with both the constitutive and the inducible form of HSP90. It does not bind to the native form and does not recognize HSP90 from E.coli or yeast. |
| Dilution | WB (1:1000), IHC (1:2000); optimal dilutions for assays should be determined by the user. |
| Storage Buffer | PBS pH7.4, 50% glycerol, 0.09% sodium azide |
| Description | HSP90 is a highly conserved and essential stress protein that is expressed in all eukaryotic cells. From a functional perspective, HSP90 participates in the folding, assembly, maturation, and stabilization of specific proteins as an integral component of a chaperone complex (1-4). Despite its label of being a heat-shock protein, HSP90 is one of the most highly expressed proteins in unstressed cells (1–2% of cytosolic protein). It carries out a number of housekeeping functions â€â-regulated proteins that have been discovered to date are involved in cell signaling (5-6). The number of proteins now know to interact with HSP90 is about 100. Target proteins include the kinases v-Src, Wee1, and c-Raf, transcriptional regulators such as p53 and steroid receptors, and the polymerases of the hepatitis B virus and telomerase (5). When bound to ATP, HSP90 interacts with co-chaperones Cdc37, p23, and an assortment of immunophilin-like proteins, forming a complex that stabilizes and protects target proteins from proteasomal degradation. In most cases, HSP90-interacting proteins have been shown to co-precipitate with HSP90 when carrying out immuno-adsorption studies, and to exist in cytosolic heterocomplexes with it. In a number of cases, variations in HSP90 expression or HSP90 mutation has been shown to degrade signaling function via the protein or to impair a specific function of the protein (such as steroid binding, kinase activity) in vivo. Ansamycin antibiotics, such as geldanamycin and radicicol, inhibit HSP90 function (7). For more information visit our HSP90 Scientific Resource Guide at http://www.HSP90.ca. |
| Reference | 1. Arlander SJH, et al. (2003) J Biol Chem 278: 52572-52577. 2. Pearl H, et al. (2001) Adv Protein Chem 59:157-186. 3. Neckers L, et al. (2002) Trends Mol Med 8:S55-S61. 4. Pratt W, Toft D. (2003) Exp Biol Med 228:111-133. 5. Pratt W, Toft D. (1997) Endocr Rev 18: 306–360. 6. Pratt WB. (1998) Proc Soc Exptl Biol Med 217: 420–434. 7. Whitesell L, et al. (1994) Proc Natl Acad Sci USA 91: 8324– 8328. |
|---|---|
| Gene Symbol | HSP90 |
| Gene Full Name | heat shock protein 90kDa alpha (cytosolic), class B member 1 |
| Alias Symbols | HSP84, HSP86, HSP90, HSP89, HSP90Beta, HSP90A, HSP90AA1, HSP90AB1, HSP90B, HSP90N, HSPC1, HSPC2, HSPCA, HSPCAL1, HSPCAL4, HSPCB, HSPN, LAP2 |
| NCBI Gene Id | 4768 |
| Uniprot ID | Q8LLI5 |
| Nucleotide Accession # | AAM90675.1 |