nanoCAR-T mRNA service

Non-viral chimeric antigen receptor (CAR)-T cells are gaining attention for their cancer-killing ability with reduced side effects. Traditional CAR-T cell engineering relies on lentivirus, which causes random chromosomal integration, permanent CAR expression, and adverse effects from persistent immunologic stimulation. To address these issues, mRNA-based CAR-T therapy offers a promising alternative by enabling temporary yet effective CAR expression in T cells.
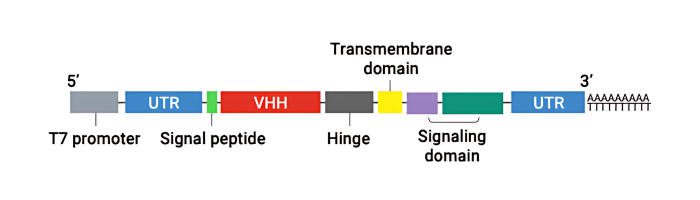
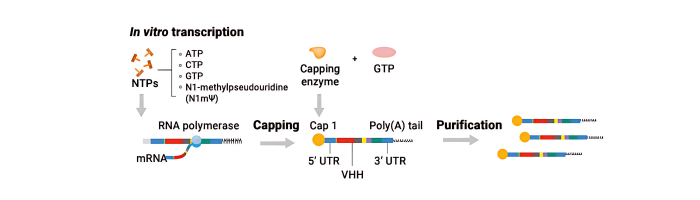
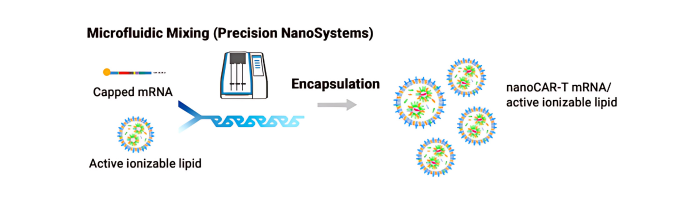
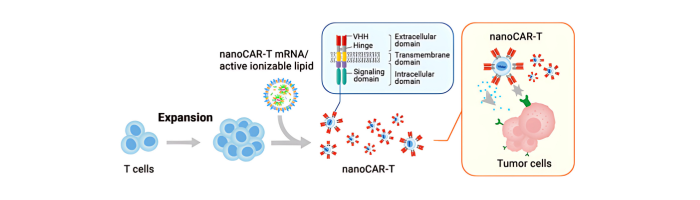
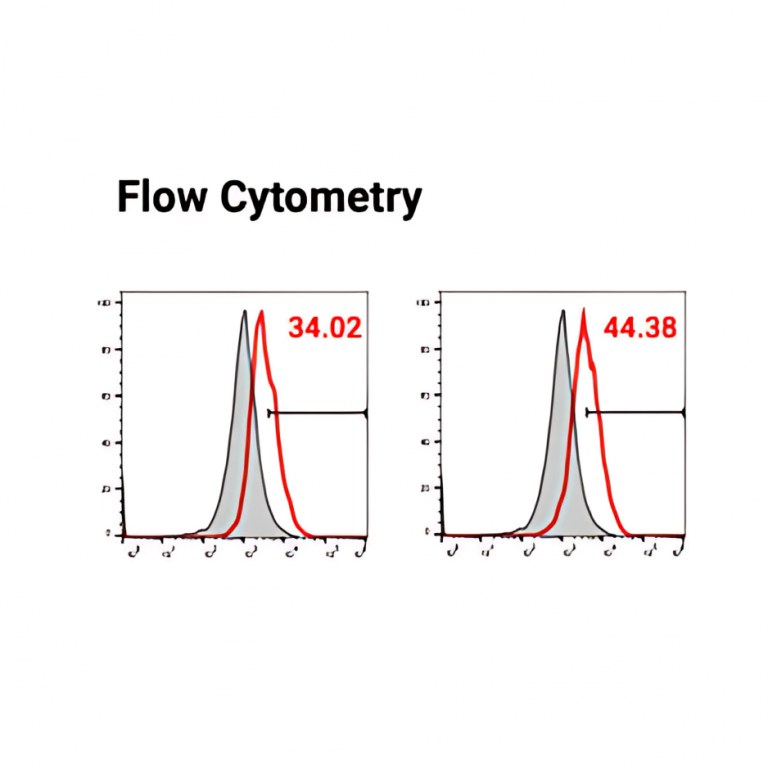
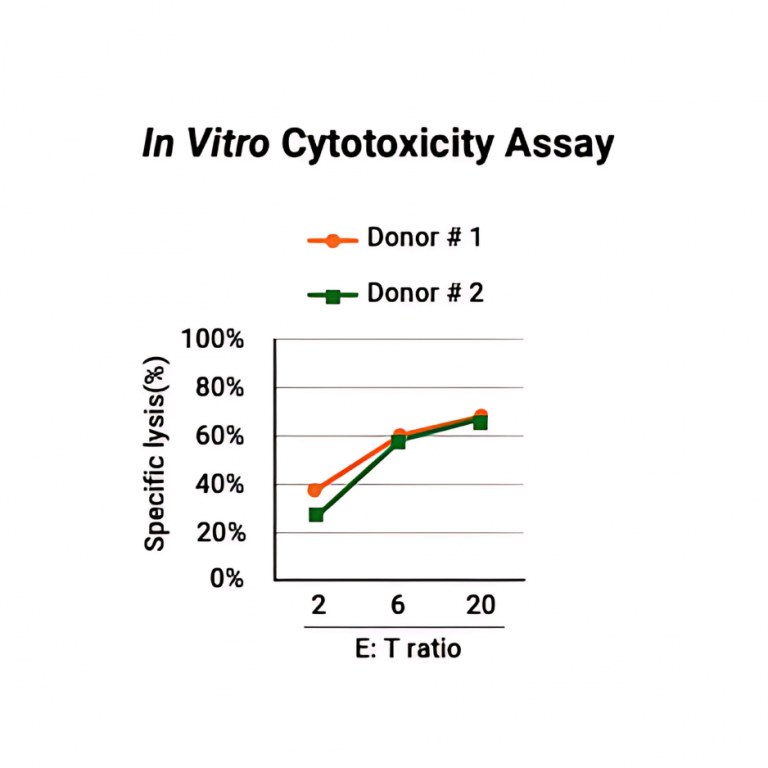
We provides a nanoCAR-T mRNA service that advances CAR-T cell therapy by integrating its NanoAb™ VHH antibody platform, mRNA IVT technology, and lipid nanoparticle (LNP) delivery. This approach allows precise ex vivo delivery of CAR instructions to T cells via mRNA encapsulated in ionizable lipid nanoparticles. Unlike conventional CAR-T therapies that use single-chain variable fragments (scFv) from monoclonal antibodies, We incorporates smaller VHH antibodies for high affinity antigen targeting. This allows access to cryptic antigen epitopes often missed by traditional antibodies and improve tissue penetration into the highly complex tumor microenvironment. The smaller VHH antibodies also enable the construction of bispecific CAR-T to expand its cancer-killing options. Workflow DNA Construct nanoCAR-T mRNA Production 1 2 Ex vivo nanoCAR-T mRNA delivery 4 Expression level of CD13 on human T cells. The cell surface expression of CD13 nanoCAR was detected by with Alexa Fluor488 AffiniPure Goat Anti-Alpaca IgG, VHH domain flow antibody. In vitro cytotoxic activity of CD13 nanoCAR-T cells THP-1 cells were incubated with CD13 nanCAR-T cells at the indicated E: T ratios for 24h. nanoCAR-T mRNA Encapsulation 3 nanoCAR-T mRNA Service In Vitro Cytotoxicity Assay Flow Cytometry With expertise not only in VHH antibodies but also in mRNA CAR-T vector design, sequence optimization, modification, and purification for expression in T cells, the nanoCAR-T service provides a flexible and scalable CAR-T production process that avoids the high costs and limitations of viral vector systems. This makes it a promising platform for applications in liquid tumors, solid tumors, and autoimmune disease research.
Advantages
- Lower Cost
- Decrease Cycle
- Time Enhanced
- Safety for Early-Stage Testing
- Avoid Chromosomal Integration
- Potential for Repeat Dosing
Workflow
|
1- DNA Construct
|
2- nanoCAR-T mRNA Production
|
|
3- nonCAR-T mRNA Encapsulation
|
4- Ex vivo nanoCAR-T mRNA delivery
|
Examples
 |
 |
|
|
Expression level of CD13 on human T cells. The cell surface expression of CD13 nanoCAR was detected by with Alexa Fluor488 AffiniPure Goat Anti-Alpaca IgG, VHH domain flow antibody. |
In vitro cytotoxic activity of CD13 nanoCAR-T cells THP-1 cells were incubated with CD13 nanCAR-T cells at the indicated E: T ratios for 24h. |
|
For more informations and quotations
please contact tech@clinisciences.com